Choosing the Right Clinical Data Management Company: Key Factors to Consider
Introduction:
Selecting the right clinical data management (CDM) company is a crucial decision for any organization involved in clinical trials. A reliable CDM company not only ensures high-quality data handling but also helps maintain compliance with regulatory standards, ultimately safeguarding the integrity of the study. With so many providers on the market, each offering a range of services and technologies, making the right choice can be challenging. This guide will outline the essential factors to consider when choosing a CDM company and the questions to ask to find the best fit for your clinical research needs.
Why a Good CDM Partner Matters
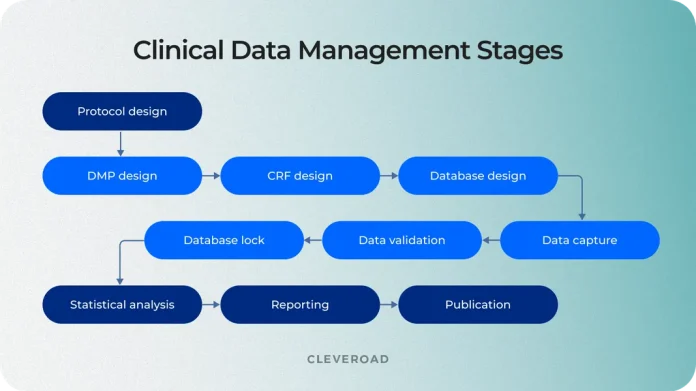
Clinical data management is central to the success of any clinical trial. CDM companies oversee the collection, cleaning, validation, and storage of data, ensuring it’s accurate, compliant, and ready for analysis. With regulatory agencies like the FDA and EMA requiring strict adherence to data quality standards, selecting a reputable CDM partner can significantly impact both the speed and reliability of your study’s outcomes. The right CDM company not only ensures data integrity but also provides peace of mind, knowing that your data is in capable hands.
Key Factors to Consider When Choosing a Clinical Data Management Company
1. Industry Experience and Expertise
Look for a CDM company with proven experience in your specific therapeutic area or type of clinical study. A company with a deep understanding of the nuances of your field is more likely to anticipate challenges, streamline processes, and provide insights that a less experienced company might miss. Experienced CDM providers also tend to have established processes that can reduce errors and improve data quality.
Questions to Ask:
- How many years have you been managing clinical data?
- What types of clinical studies do you have experience with?
- Can you provide case studies or references in our therapeutic area?
2. Technology and Data Management Systems
A modern CDM company should use advanced electronic data capture (EDC) systems and data management technologies that facilitate real-time data tracking, improve accuracy, and enhance efficiency. Additionally, systems should have robust security features to ensure data privacy and integrity. Consider asking about their technology stack, including whether they use cloud-based systems, mobile data collection, and integration with other software like CTMS (Clinical Trial Management Systems).
Questions to Ask:
- What EDC systems and other technologies do you use?
- How do your systems ensure data security and compliance?
- Can your system handle multi-site and multi-national data collection?
3. Data Quality and Accuracy Standards
High-quality data is the foundation of any clinical study, so it’s essential to choose a CDM company with rigorous data validation and quality control processes. Look for companies that emphasize data cleaning and validation to minimize errors and discrepancies, ensuring that your data is reliable and ready for analysis.
Questions to Ask:
- What quality control measures do you have in place?
- How do you handle data discrepancies or missing data?
- How often do you perform data audits or quality checks?
4. Compliance and Regulatory Knowledge
Regulatory compliance is critical in clinical research, especially regarding data handling. The CDM company you choose should be well-versed in regulatory requirements, including those set forth by the FDA, EMA, and ICH GCP (Good Clinical Practice). Compliance with standards such as 21 CFR Part 11 (for electronic records) is essential for securing regulatory approval and ensuring that your study adheres to the latest guidelines.
Questions to Ask:
- Are you compliant with FDA, EMA, and other relevant regulatory standards?
- Do your systems meet 21 CFR Part 11 compliance for electronic records?
- How do you stay up-to-date with evolving regulatory guidelines?
5. Data Security and Confidentiality Measures
Ensuring data security is paramount in clinical research, where sensitive patient information is often collected. Choose a CDM company that follows strict data security protocols, including data encryption, secure access controls, and regular security audits. Compliance with data protection regulations like GDPR (General Data Protection Regulation) is also essential, especially for studies involving international data.
Questions to Ask:
- What data security measures do you have in place?
- Are you compliant with GDPR and other data protection regulations?
- How do you handle data breaches, if they occur?
6. Flexibility and Scalability of Services
The scope of clinical trials can change over time, especially for multi-phase studies or those expanding to multiple sites. It’s important to choose a CDM company that can scale its services and adapt to your changing needs, whether that means handling larger datasets, accommodating new data sources, or managing complex, multi-site trials.
Questions to Ask:
- Can you scale your services to accommodate larger or multi-site trials?
- How do you handle changing data requirements over time?
- What flexibility do you offer in terms of service customization?
7. Cost and Value for Money
While cost should not be the sole deciding factor, understanding the pricing structure and the value provided by the CDM company is essential. Some companies charge a flat fee, while others may charge based on the number of participants or sites. Ensure that the pricing aligns with your budget and that the services provided justify the cost.
Questions to Ask:
- What is your pricing structure, and what factors affect the cost?
- Are there additional fees for data storage, reporting, or data access?
- How do you ensure that your services provide value for money?
8. Customer Support and Responsiveness
Clinical trials are time-sensitive, and delays in data management can have significant implications. Choose a CDM company that offers responsive customer support and has a reliable communication process. A dedicated project manager or point of contact can be beneficial for quickly addressing issues and keeping your study on track.
Questions to Ask:
- What customer support options do you offer?
- Will we have a dedicated project manager or point of contact?
- How quickly can you resolve issues or respond to queries?
Additional Tips for Choosing a CDM Company
- Ask for References: Speak to other clients who have worked with the CDM company, particularly those with similar study requirements. This can provide valuable insights into the company’s performance, reliability, and support quality.
- Request a Trial or Demo: Many CDM companies offer trials or demos of their EDC systems and other tools. Requesting a demo can help you evaluate the user experience and assess whether the system meets your team’s needs.
- Evaluate Their Training and Support: A good CDM partner should offer training to ensure your team can effectively use their systems. This is particularly valuable if your team has limited experience with EDC systems or data management protocols.
- Assess Cultural Fit: If your trial is long-term, a good working relationship with the CDM company is essential. Consider whether their team shares your values, priorities, and approach to problem-solving.
Choosing the right clinical data management company is a critical decision that can impact the success of your clinical study. By evaluating factors such as industry experience, technology, data quality standards, compliance, security measures, and customer support, you can identify a CDM partner that aligns with your needs and goals. Remember that the right CDM company does more than manage data; it becomes a strategic partner, enhancing the reliability, compliance, and overall success of your clinical research. Taking the time to carefully select your CDM provider is an investment in the future of your study and, ultimately, in the advancement of healthcare.